ABOUT IABS
The International Alliance for Biological Standardization (IABS) is an independent, non-profit scientific alliance, with headquarters in Geneva (Switzerland).
IABS was founded in 1955 in Lyon, France by a group of experts who believed that such an organization could provide a forum where data could be discussed among scientists to improve the quality and the regulation of biological products from human and animal origin. IABS has members in over 50 countries.
Mission
The mission of the Alliance which is related to the scientific and medical advancement of Biologicals, includes :
- To host conferences and workshops, facilitate communication, and develop consensus on key contemporary issues related to biologicals among those who discover, develop, produce and regulate biological products for human and animal health
- To foster evidence-based Science as the standard for improving and ensuring the quality and the safety of biological products
- To share scientific advancements and innovative regulatory solutions through our journal “Biologicals”.
Organization
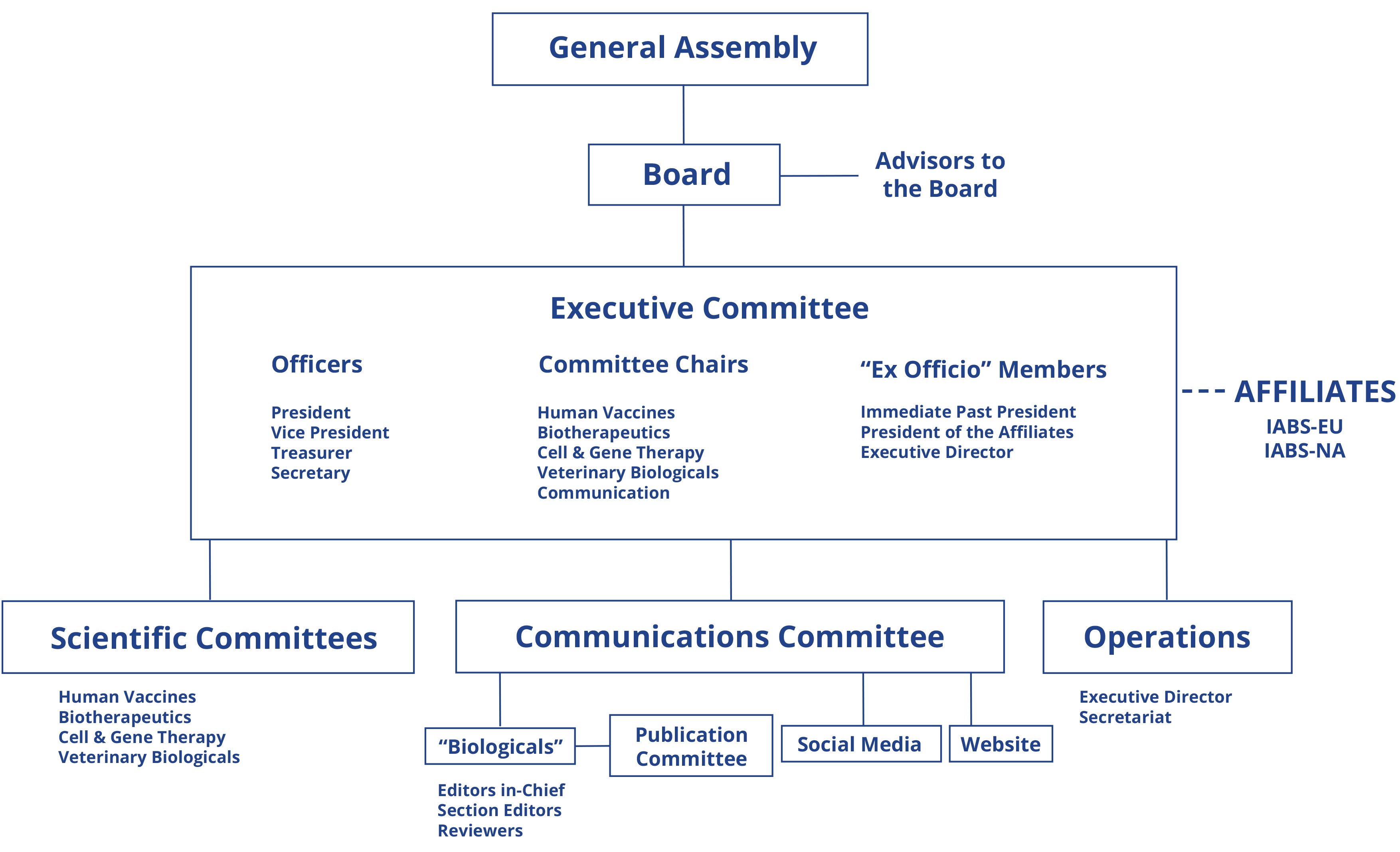
- The General Assembly represents the ultimate authority of the Alliance. It meets every two years and is constituted by the membership.
- The Board which meets once a year, is responsible for ensuring that
– The Alliance’s goals are respected
– The Constitution and the Conflict of Interest policy are properly applied
– The Alliance’s assets, including its income, are adequately employed - The Scientific Committees who are responsible for organizing meetings across and within their respective domains.
- The Alliance’s Communications Committee is responsible for all publication activity, including managing and reviewing articles for its journal Biologicals, and coordinating information to be published in social media and on the IABS website. The Committee also helps to ensure that contacts with selected partner organizations remain active.
- The Affiliates in Europe (IABS-EU) and North America (IABS-NA) who support the needs and expectations in their regions.
The Value of IABS
- Four fields of expertise : Human Vaccines, Biotherapeutics, Cell & Gene Therapy, Veterinary Biologicals.
. - Strengths of IABS Scientific Events : IABS’ unique role and strength reside in its ability to bring together interested parties, as a neutral facilitator, for scientific discussions of important unresolved or emerging issues, to assist in developing a consensus and an action plan to achieve regulatory progress. This strategy has led to significant gains in both the human and veterinary fields, and it differentiates IABS conferences from most scientific meetings that focus simply on the exchange of information. Another unique aspect of IABS conferences is the final session which is dedicated to drafting consensus meeting summaries, conclusions & recommendations which are subsequently made available interested parties such as regulatory agencies and the biological and biopharmaceutical industries. In many cases, these recommendations have had a positive impact on global regulatory processes.
. - IABS’ Journal, “Biologicals” includes both foundational scientific papers and summaries of our scientific events.
. - Experience : Since IABS was created in 1955, participants from around the globe have attended more than 170 IABS Scientific Events (meetings, conferences and workshops).
Projects
To learn more about IABS projects click here.